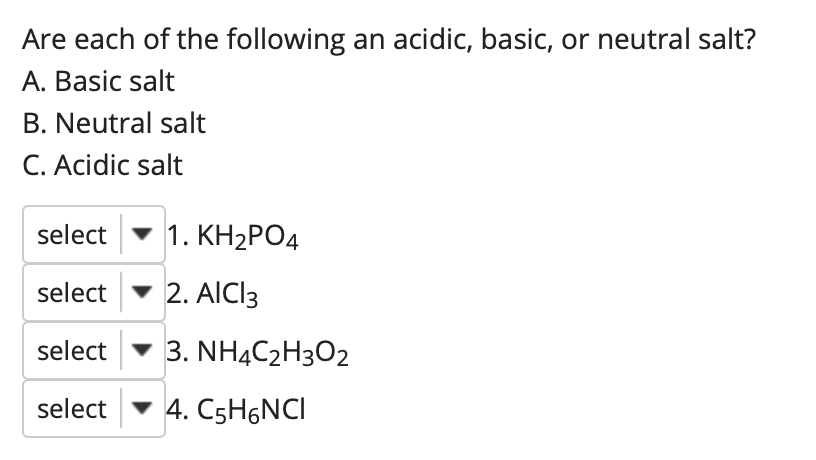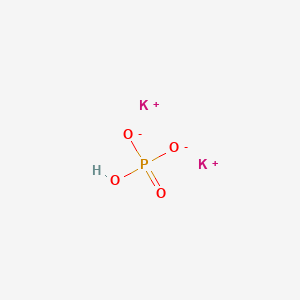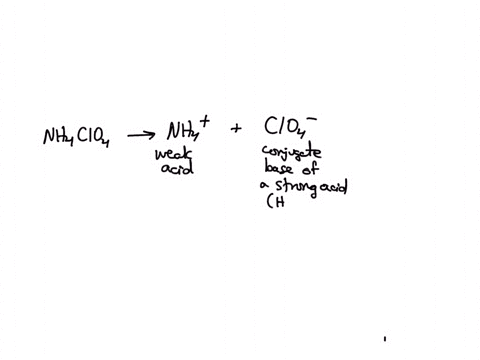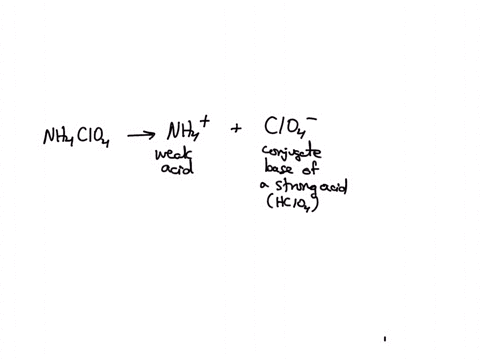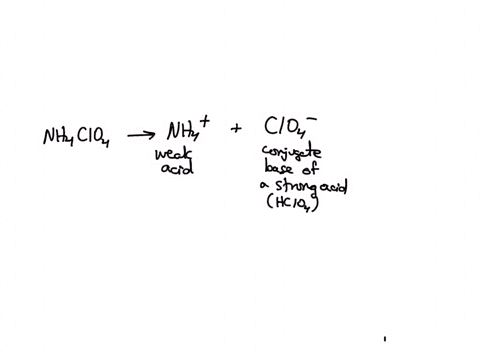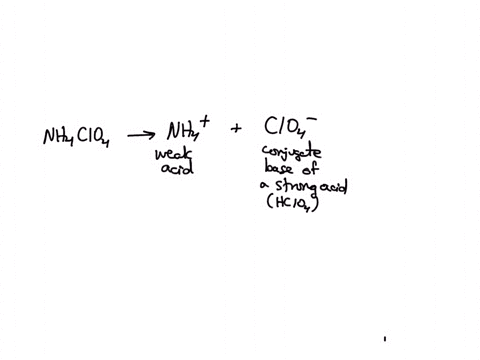
SOLVED: Polyprotic Acids (1) Is Dipotassium phosphate acidic, basic or neutral? (2) Is monopotassium phosphate (also called Potassium dihydrogen phosphate) acidic, basic or neutral? (3) Is Disodium citrate acidic, basic or neutral?

If I want to make a potassium phosphate buffer solution, could I use KOH and H3PO4 instead of KH2PO4 and K2HPO4? | ResearchGate

Enhanced Second-Harmonic-Generation Response in a KH2PO4-Type Calcium Nitrate Carboxylate with Unusual Three-Dimensional Inorganic and Organic Connections | Inorganic Chemistry
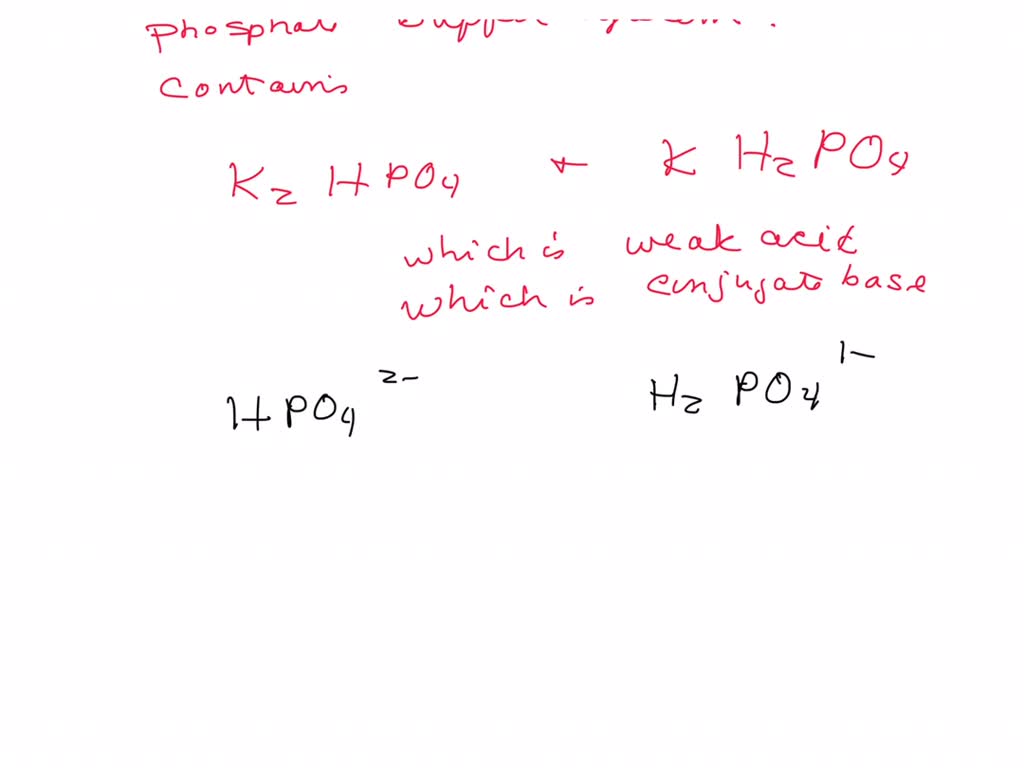
SOLVED: In the phosphate buffer system containing K2HPO4 and KH2PO4, what is the weak acid? What is its conjugate base?
![Potassium Phosphate Monobasic (KH2PO4, 500g) [CK02-500G] - $30.00 : Bioland Scientific, for Your Research Needs Potassium Phosphate Monobasic (KH2PO4, 500g) [CK02-500G] - $30.00 : Bioland Scientific, for Your Research Needs](https://www.bioland-sci.com/images/KH2PO4%20500G.jpg)
Potassium Phosphate Monobasic (KH2PO4, 500g) [CK02-500G] - $30.00 : Bioland Scientific, for Your Research Needs
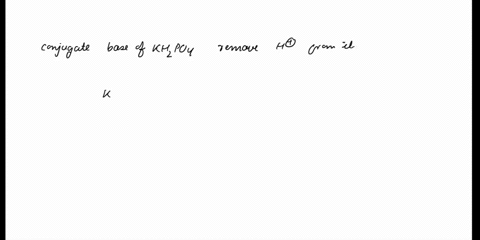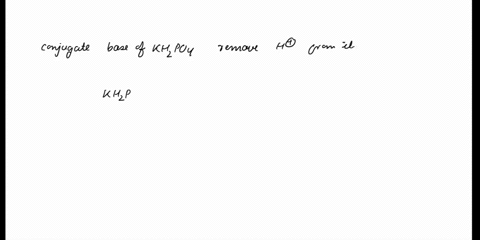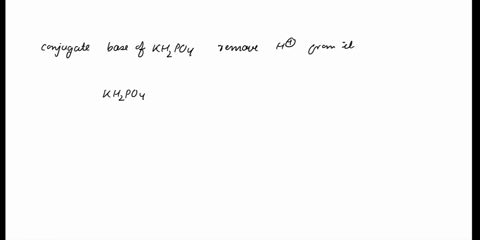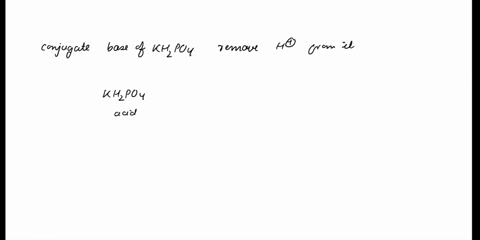
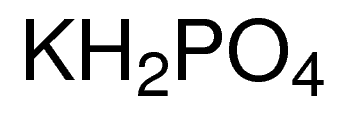



(358).jpg)